Figure 1:


Hot Dip Galvanising
How Galvanising prevents corrosion of steel: -
| Hot dip galvanizing is a surface treatment of steel that uses zinc as coating material. Zinc coating being electrically more active than steel, acts as a sacrificial anode layer. (See Figure 2 and 3 ) This inhibits corrosion of steel, even when the coating is scratched. |
|
Figure 1: |
|||
 |
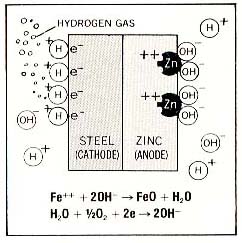
Similar to corrosion of steel ( see Corrosion of Steel ) , Zinc being more electrolytic-ally active corrodes in place of the steel layer. | ||
| Figure 2: | |||
 |
Zinc looses 2 electrons to steel because steel is the cathode, and is electrically attractive. | ||
| Figure 3: | |||
 |
Zinc forms zinc oxide and water. Hydrogen gas is released in the process. | ||
| Figure 4: | |||
 |
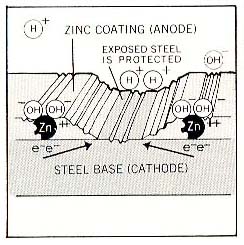
In event of a scratch, steel may be exposed. The zinc coating becomes an anode and protects the base metal by corroding first. This inhibits the exposed steel from corroding. ( see Figure 4 ). | ||
| Pictures from ZALAS handbook |
Corrosion of Steel: -
|
We often see Steel ( Iron ) as simply rusted but it is actually 3 different chemical reactions happening simultaneously. Iron is an electropositive metal
and forms cathode-anode cells that react with the electrolyte, in most cases moisture or water.
|
|
| Corrosion starts at the surface of iron, where differences in potential exist, because of impurities. This non-uniformity causes Iron to losses 2 electrons to the cathode that is electrically positive: - | |
|
Fe
|
|
| Electrons migrate from surface of Iron to the cathode part of the metal. The depleted iron combines with 2 units of OH- becoming iron oxide ( rust ) and water: - | |
|
Fe++ + 2OH-
|
|
| 2 units of H+ ions combine with free electrons from Iron to become hydrogen gas:- | |
|
2H+ + 2e-
|
|
Process of Hot Dip Galvanising: -
|
|
|
|
( Picture from Industrial Galvanisers Corporation Australia ) |
|
|
During Hot Dip Galvanising, the steel surface is dipped into molten zinc, and a protective layer of zinc-iron alloy and pure zinc form quickly to seal it from corrosion. Galvanising is divided into 3 main steps. Surface Preparation, Fluxing and Galvanizing. |
|
|
Surface Preparation: - Hot Caustic ( alkaline ) cleaner is used to remove oil, grease and soluble paints. Subsequently, acid cleaning removes surface rust and contaminants. Steel castings are abrasive blast cleaned, followed by brief acid cleaning or electrolytic cleaning. Then the work is rinsed with water to get rid of acids prior to fluxing. |
|
|
Fluxing of steel: - completely immerse the steel in a solution of 30% zinc ammonium chloride + wetting agents. Molten flux is maintained at 440°C to 460°C. Fluxing protects the steel surface prior to galvanising. |
|
|
Galvanising: - the steel is later immersed in molten zinc at 445°C to 465°C. It remains in the bath until temperature reaches that of the molten zinc. Finally, the work is withdrawn at a control rate and quenched in Chromate, forming a shiny layer of pure zinc, characterised by a spangled pattern. ( Refer to Inspection of Surface Characteristics --> Dull grey coating ) |
|
Inspection of surface characteristics: -
|
The quality of galvanising process can be confirmed visually. At a glance it is easy to identify problems with the coating. We can also use magnetic gauges that provide simple non-destructive testing methods for coating thickness. The following table shows the surface finish in relation to acceptability.
|
| Surface finish | Picture | Description | Condition |
| Dull grey coating |  |
Due to thicker zinc-iron alloy | Acceptable |
| Rust stains |  |
Due to contact with rust from other corroded steels | Acceptable when present as surface stain |
| Lumpiness and runs |  |
Due to uneven drainage of molten zinc | Acceptable |
| Bare spots |  |
Due to air pockets or under preparation surface, and number of factors beyond control | Acceptable if area is small |
| Roughness |  |
Thicker than normal coatings | Acceptable |
| Dark spots/ flux staining/ ash inclusions |  |
By product of the galvanizing process | Acceptable if removal reveals sound coating |
| Pimples |  |
Due to incursion of impurities into coating | Not Acceptable |
| Wet storage stain or bulky white deposit |  |
Closely stacked articles become damp under poor ventilation during storage or transportation |
Acceptable if deposit is removed and coating thickness is not substantially thinner. |
| Blisters | Due to absorbed hydrogen expelled during galvanizing, extremely rare |
Small intact blisters acceptable |
Pictures from ZALAS handbook
Standards: -
The most commonly used Standards in Singapore are : -
British Standard
| BS 729 | - | Hot-dipped galvanised coatings on iron and steel articles |
| EN ISO 1461:1999 | - | Hot-Dipped Galvanised coatings on fabricated |
| iron and steel articles - Specifications and test methods |
American Standard
| ASTM A 123 |
- |
Zinc (Hot galvanised) coatings on products fabricated from rolled, |
| pressed, and forged steel, steel shapes, plates, bars, and strips | ||
| ASTM A 143 |
- |
Recommended practice for safeguarding against embrittlement of |
| Hot-Dip Galvanised structural steel products and procedure for | ||
| detecting embrittlement | ||
| ASTM A 153 |
- |
Zinc coating (Hot-Dip) on iron steel hardware |
| ASTM A 384 |
- |
Practice for safeguarding against warpage and distortion during |
| Hot-Dip Galvanising of steel assemblies | ||
| ASTM A 385 |
- |
Standard practice for providing high quality zinc coatings (Hot-Dip) |
| ASTM A 767 |
- |
Standard specification for zinc coated (galvanised) steel bars for |
| concrete reinforcements |
References: -
1) ZALAS ( Zinc and Lead Asian Services ) Handbook
2) Super Galvanising Singapore website: http://www.supergalvanising.com/main.html
3) Industrial Galvanizers Australia website: http://www.corp.indgalv.com.au/technical/manual.htm
4) Rust: - How Stuff Works: http://science.howstuffworks.com/question445.htm/printable
5) http://www.azom.com/details.asp?ArticleID=1075
6) http://www.nkcoatings.com/service